A review study with the title ’Silver nanoparticles as potential antiviral agents’ is published in the journal Molecules. The article details how nanosilver particles exert their antiviral effects on seven examples of viruses.
Scientific studies to date have clearly demonstrated that nanosilver particles are extremely effective in destroying a variety of viruses. Studies have been performed with human immunodeficiency virus type 1 (HIV-1), herpes simplex virus type 1 (HSV-1), respiratory syncytial virus (RSV), monkeypox virus, Tacaribe virus (TCRV) and hepatitis B virus (HBV).
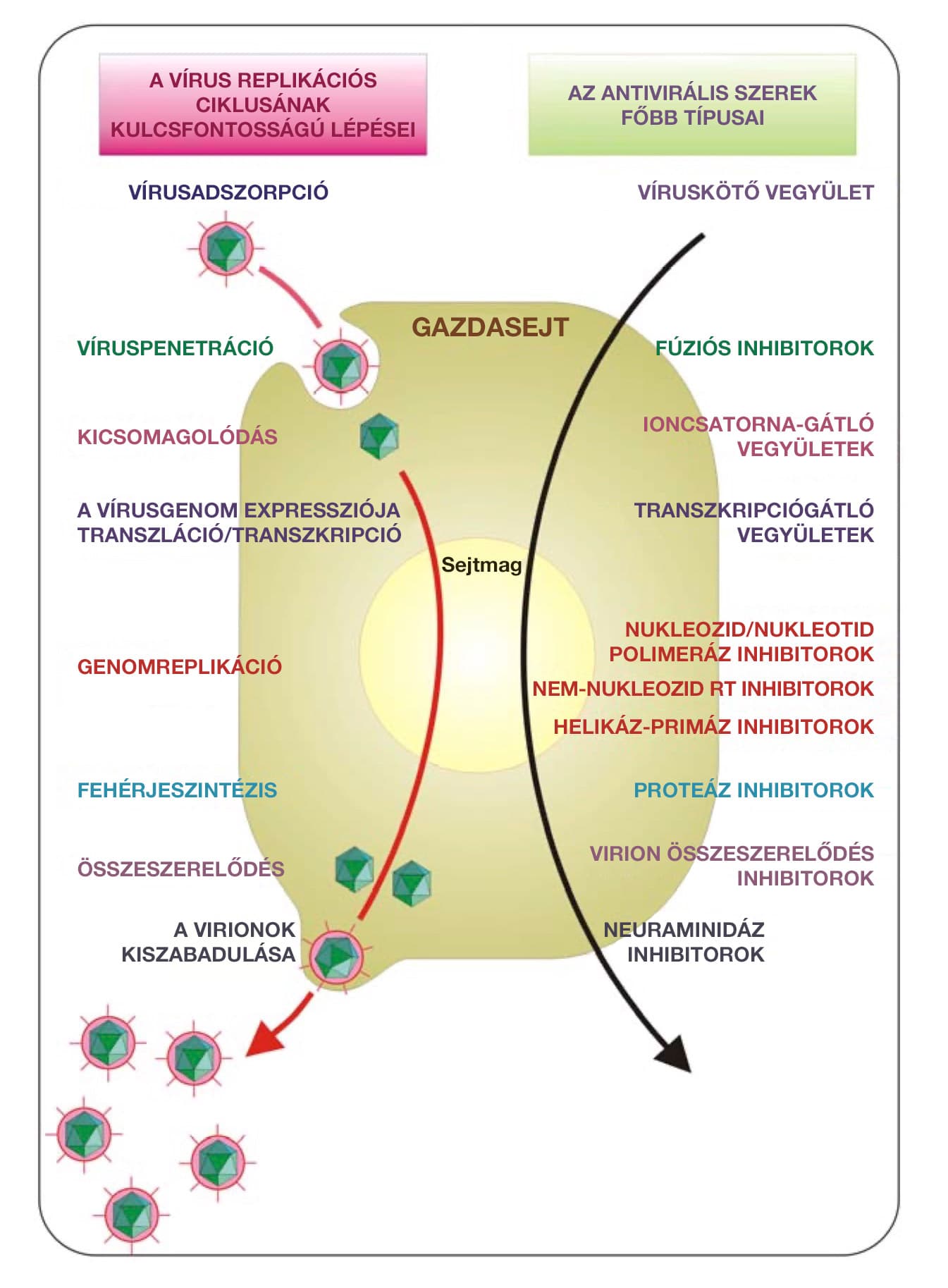
This is important because viruses can become resistant to conventional antiviral therapies that have been applied for a long time, so there is a great need for new antiviral therapies that can be used even when conventional antiviral therapies are no longer effective. The use of metal nanoparticles in the development of antiviral therapies also open up new perspectives: metals can attack multiple targets of viruses, thus reducing the risk of developing viral resistance to treatment compared to conventional antiviral therapies.
How does nanosilver work virucide?
For each of the seven viruses mentioned, Stefania Galdiero et al. describe in detail the mechanism by which silver nanopaerticles are able to neutralize viruses.
Human immunodeficiency virus 1(HIV-1)
Scientific studies with human immunodeficiency virus type 1 (HIV-1), which is responsible for the development of AIDS, have shown that silver nanoparticles bind mostly to specific parts of the HIV viral gp120 glycoprotein, and are therefore able to inhibit the initial steps of the infection cycle of HIV-1.
Researchers also showed that silver nanoparticles inhibit the entry of the virus into CD4 cells by inhibiting the gp120-CD4 interaction (CD4 cells are so-called helper T cells that are actively involved in destroying viruses and are targets of the HIV virus).
Research has also revealed that silver nanoparticles may not only bind to gp120, but may also inhibit other proteins of HIV virus, and may reduce HIV-specific reverse or proviral transcriptional rates by binding directly to the RNS or DNS molecules of the HIV virus. (The HIV genome is a single-stranded RNA and the viral particle is surrounded by a lipid envelope. Once it enters the host cell, the RNA genome is transcribed by the viral reverse transcriptase enzyme into double-stranded DNA, which is then integrated into the host cell’s own DNA.)
The virucidal activity of silver nanoparticles has been demonstrated for both cell-free and cell-bound HIV-1 viruses. The infectivity of HIV has passed since the isolated virus was exposed to silver nanoparticles, the authors write.
Herpes simplex virus 1 (HSV-1)
The responses of herpes simplex virus 1 (HSV-1), a double-stranded DNA herpesvirus, to nanosilver have been described by the researchers as follows.
The virus uses five types of glycoproteins (gB, gC, gD, gH and gL) to enter the cells it wants to infect.
Studies have shown that silver nanoparticles prevent it from binding to cells with its glycoproteins, so that the virus cannot infect selected cells from the outset. It has also been shown that nanoparticles can also inhibit the spread of the virus from cell to cell.
Respiratory syncytial virus (RSV)
Respiratory syncytial virus is a single-stranded RNA virus that infects the epithelial epithelium of the lungs and airways, causing severe respiratory problems, especially in children and the elderly. There is no vaccine or effective drug against the virus, so it would be essential to develop some new drugs in the future.
Transmission electron microscopy images of the interaction between nanosilver and virus particles showed that nanoparticles coated with bovine serum albumin (BSA) contacted the virus, but the binding did not show any regular spatial pattern. Silver nanoparticles coated with polyvinylpyrrolidone (PVP) also contacted the virus, and binding in this case showed a regular spatial pattern suggesting that these nanoparticles are able to bind to G proteins present in a uniform distribution on the surface of RSV virions. BSA and PVP coatings were needed to reduce the toxicity of nanosilver particles.
The antiviral efficacy of the nanoparticles was determined by immunofluorescence microscopy: experts found that BSA-coated nanoparticles did not inhibit the spread of RSV infection in cells, whereas PVP-coated particles reduced the rate of virus spread in cells by 44 percent. The inhibition is caused by binding to G proteins, which prevents the virus from entering the cells.
Hepatitis B virus (HBV)
Hepatitis B viruses partly contain double-stranded DNA surrounded by a lipid envelope. HBV attacks liver cells (hepatitis) and once it enters the cell, the virus particles head toward the nucleus where the viral genome is converted to covalently closed circular DNA (cccDNA).
Currently available antiviral agents target primarily the viral polymerase reverse transcriptase enzyme: although these agents have been shown to be effective, the number of HBV strains resistant to them has risen sharply, limiting their use.
A group of researchers analyzed the ability of monodisperse silver nanoparticles to inhibit the ability of HBV to reproduce. Nanoparticles of several sizes were used in the study: 800 nanometer particles (Ag800Ns) were found to be too toxic to be used as antiviral agents, while 10 and 50 nanometer particles (Ag10Ns, Ag50Ns) were already usable as they showed minimal toxicity at that concentration, which was required to inhibit viral replication.
The researchers reported that both size types of nanoparticles showed significant antiviral activity: for Ag10N, this meant 38 % inhibition of viral replication at 5 µM concentration and 80 % inhibition at 50 µM concentration. Ag50N showed 53 % inhibition of viral replication at 5 µM and 92 % inhibition at 50µM concentration.
Researchers hypothesize that silver nanoparticles inhibit the production of RNA molecules and extracellular virions required for HBV replication by binding to and/or directly binding to viral double-stranded DNA.
Influenza virus
This virus causes recurring epidemics every year as it is a highly infectious pathogen with 8 RNA segments present in its helical capsid. The capsid is surrounded by a lipid envelope containing 2 types of prickly protruding glycoproteins, hemoagglutinin A (HA) and neuroaminidase (NA).
The virus is able to bind to lipids on the plasma membrane of the cells to be infected through an interaction between hemoagglutinin A and sialic acid (SA) on the glycoproteins and the plasma membrane of the cells to be infected, which is mediated by the intracellular receptor for the virus.
The researchers used gold nanoparticles instead of silver, which were provided with a sialic acid-terminal glycerol dendron to prevent the virus from binding to the plasma membrane.
Of the gold nanoparticles, the 2-nanometer and 14-nanometer versions were tested and found that the 2-nanometer version had no effect on hemoagglutination, while the 14-nanometer version successfully inhibited the process of binding of virus particles to the cell membrane (nanomolar concentration).
Simian smallpox (MPV)
Simian smallpox (or monkeypox) that is similar to variola virus can infect non-human anthropoid races, but it is also dangerous to humans, and causes anthrax-like, life-threatening symptoms.
The research also involved silver nanoparticles of various sizes, which were added to the viruses at concentrations ranging from 12.5 to 100 micrograms / millilitre.
The results showed that the 25-nanometer polysaccharide-coated silver nanoparticles exerted significant dose-dependent inhibition of smallpox virus from forming plaques. (the exact mechanism of this has not yet been investigated.)
Tacaribe virus (TCRV)
Although Tacaribe virus, which belongs to the Arenaviridae family, is not a human pathogen, it is closely related to human pathogens (Junin and Guanarito viruses) that can cause serious diseases in humans (such as bleeding fever). The Tacaribe virus is therefore a perfect model virus to study the properties and behaviour of the other two viruses, which are also dangerous to humans, without the risk of human infection.
The researchers used two types of silver nanoparticles in their studies, one without a polysaccharide coating and one with a polysaccharide coating.
It was found that when 10 nanometer uncoated silver nanoparticles were applied to the virus at concentrations of 50 μg/ml, 25 μg/ml and 10 μg/ml, there was a significant decrease in viral titer on all three occasions.
Similar results were observed for silver nanoparticles with a polysaccharide coating, albeit with only a small decrease in titer but lower toxicity.
The researchers hypothesize that silver nanoparticles are able to bind to viral membrane glycoproteins and thus inhibit viral replication.