WITH NANOTECHNOLOGY AGAINST INFECTIONS
About silver particles
Today’s state-of-the-art science is nanotechnology, the achievements of which are now appearing in many industries. The word nano is of Greek origin, meaning dwarf. In the system of units of measure, the nanometer is one millionth of a millimeter, i.e. 0.000000001 meters. When we talk about particles, we have to think in the world of atoms and molecules. It is really difficult to imagine the small size of nanostructured materials. But to get an idea of these dimensions, here is an example: the size of a particle relates to a soccer ball in the same way that a soccer ball relates to the Globe.
The question may rightly arise as to what can be used for tiny devices, objects, the dimensions of which can be measured with the length of one millionth of a millimeter.
The answer is that the goal of nanotechnology is to produce materials and devices that are useful to humanity within the nanometer range. To better understand this, we need to know that the physical and chemical properties of all materials differ significantly from those of the same material when the material is broken down into nanoparticles. Silver is an excellent example to illustrate this.
Silver particles have outstanding antibacterial properties, able to kill hundreds of bacterial species in a matter of minutes. The effect of silver particles on bacteria and the cause of it is still being studied today, every detail of the mechanism of action is an exciting research topic in most scientific workshops around the world.
According to an accepted theory, the antibacterial effect of silver particles can be attributed to the increased activity in chemical reactions. Briefly, when a bacterium and a silver particle the size of a few nanometers meet, the compounds that make up the cell wall of the bacterium react with the silver particle, resulting in the release of silver ions from the surface of the particle. During this time, the compounds themselves are converted. Because the building blocks of the cell wall are transformed during the chemical reaction, the cell wall, which protects the bacterium, is damaged, so that silver ions can easily get inside the bacterium. Silver ions bind to enzymes that are vital to the bacterium and catalyze the metabolic processes of the microorganism. However, the enzymes of the pathogen are no longer able to perform their function after this chemical transformation. The death of bacteria is thus the result of a chemical reaction between silver ions and bacterial enzymes.
Reducing the size of silver particles increases their efficiency in the chemical processes described above.
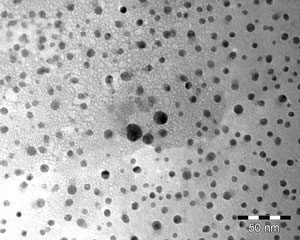
Thanks to the high level of professional work, a significant proportion of the silver particles in the Nanosept® disinfectant have a diameter of less than 20 nm. The product has been tested on a number of bacterial species and we have found that these particles can kill even the most dangerous species that are the most common causes of infections.
It has been shown in microbiological studies to be effective against, among other things, the infamous Staphylococcus Aureus, which is the cause of most fatal diseases, and also effectively kills the bacterium E. coli, which is also a common microorganism that causes infection.
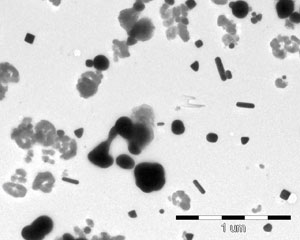
The researchers also managed to find that precipitation does not occur and that the particles do not stick together even in the presence of a significant concentration of nanosilver. This is due to the fact that a special coating has been formed around the nanoparticles, which, however, does not inhibit the disinfecting effect.
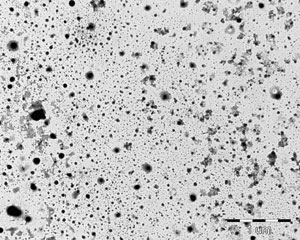
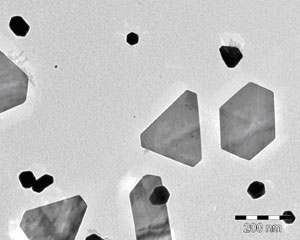
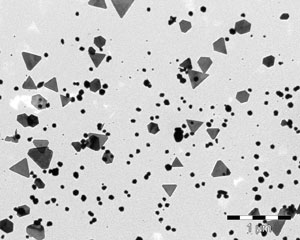
The quality of the product is checked on a system. The silver particles in the disinfectant are shown in the below electron micrographs.
The images show the varied morphology of the particles, which, when examined by electron microscopy, reveal a special world.